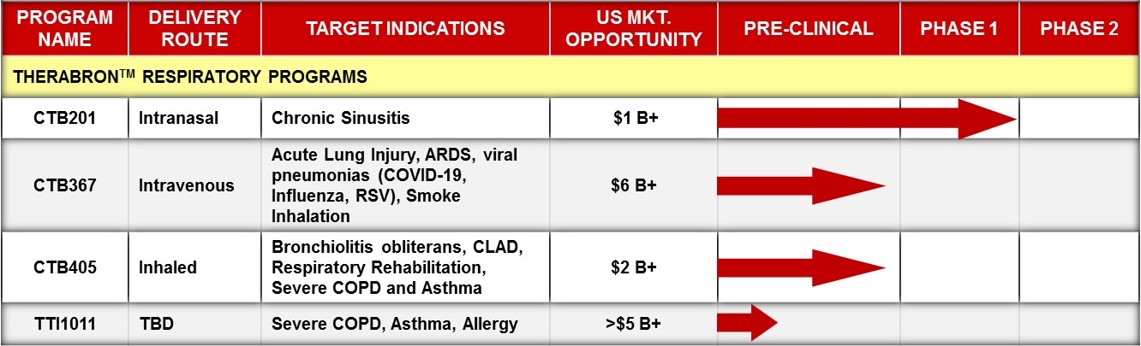
Product Candidates
Trove Therapeutics’ lead biopharmaceutical product candidate is a proprietary preparation of recombinant human SCGB1A1 protein called Therabron™, which has potential clinical indications in several respiratory diseases and conditions, including recurrent and chronic sinusitis, acute lung injury, ARDS, and viral pneumonia due to COVID-19, influenza, RSV, and other respiratory viruses, smoke inhalation, and bronchiolitis obliterans in lung and hematopoietic cell transplant recipients.
Clinical Indications for Therabron™
Recurrent and Chronic Sinusitis
An estimated 14.6% of the adult and pediatric US population is diagnosed with sinusitis in the US each year; almost 50 million people. Estimates between 5%-12% suffer from chronic rhinosinusitis (CRS); that is, 16 and 39 million people. CRS is preceded by recurrent episodes of acute sinusitis (RAS) that result in progressive fibrosis and scarring of the nasal and sinus epithelium. CRS is defined as having continuous sinusitis symptoms for 12 consecutive weeks without relief despite medication. CRS patients often have some type of airway obstruction due to the presence of nasal polyps or nasal epithelial scarring. The standard of care for patients with persistent sinusitis includes treatment with oral antibiotics and nasal corticosteroids, however, as the disease progresses, these treatments often become ineffective. Sometimes oral steroids are prescribed in cases where sinus symptoms and inflammation in the nasal cavity are particularly severe. Symptoms include sinus pain and pressure, discolored nasal drainage, postnasal drip, headache, nasal congestion, and sleeplessness. These symptoms typically recur within 1-4 weeks following completion of antibiotic treatment. Patients with RAS may have minor anatomical aberrations that are not severe enough to obstruct nasal airflow or administration of intranasal drugs, while patients with CRS often have severe remodeling of nasal epithelium with scarring. A significant proportion of CRS patients have nasal polyps that occlude airflow and must be removed surgically. The SCGB1A1 protein is normally produced by the nasal epithelium. Both SCGB1A1 and the nasal epithelial cells that produce it are depleted in CRS and are completely absent in nasal polyps. Replacement/augmentation of SCGB1A1 with Therabron™ decreases inflammation and fibrosis of the nasal epithelium in models of chronic sinusitis.
CLAD and BOS
Bronchiolitis obliterans syndrome (BOS) is a type of Chronic Lung Allograft Dysfunction (CLAD) that affects the majority of lung transplant recipients within a few years of transplant. CLAD and BOS were formerly known as chronic lung transplant rejection. CLAD-BOS is characterized by an immune-mediated progressive fibrosis and occlusion of the small airways of the lungs, by development of donor-specific antibodies (DSA) and donor-antigen specific T cells, and by declining lung function (measured by spirometry). Respiratory failure of the graft occurs within 5-6 years of CLAD/BOS diagnosis in 50% of patients, resulting in death unless a second lung transplant is performed. No therapeutics have been approved to treat or prevent CLAD/BOS. About 5-6,000 lung transplants are performed each year in the US and approximately 22,000 lung transplant recipients are currently living in the US, with similar numbers in Europe. BOS also develops in about 10% of allogeneic hematopoietic cell transplants (HCTs), of which there were a projected 97,000 living in the US in 2021. Thus, a total of about 120,000 Americans are affected by BOS.
Club cells and native SCGB1A1 protein are significantly depleted as small airway fibrosis and CLAD-BOS progresses. This depletion leaves the lungs highly susceptible to infection and injury, with lower capacity to repair damage and recover fully. Replacement/augmentation of the depleted SCGB1A1 with Therabron™ decreases pulmonary airway inflammation and fibrosis, prevents loss of lung function and body weight, decreases DSA titers, facilitates airway epithelial repair, and improves clearance of infection in models of CLAD, pulmonary fibrosis, airway epithelial ablation, and viral infection.
Trove and its collaborators plan to initiate a Phase 1 trial in CLAD patients using inhaled Therabron™ in late 2022.
ALI/ARDS, Viral pneumonias (COVID-19, Influenza, RSV), Smoke Inhalation
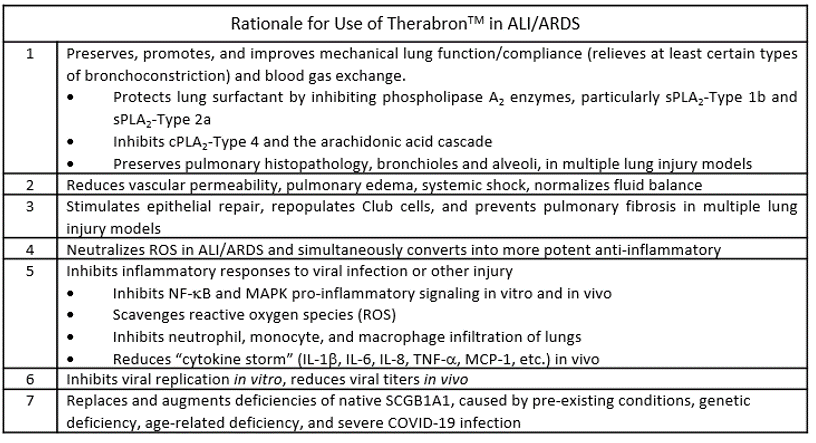
Acute respiratory distress syndrome (ARDS) is clinically defined as hypoxemia defined by a PaO2/FiO2 ratio ≤200 mmHg while acute lung injury (ALI) is only slightly less severe hypoxemia with a PaO2/FiO2 ratio ≤300 mmHg. ARDS is often preceded by ALI and both represent the acute onset of respiratory failure. ALI/ARDS also display bilateral infiltrates on chest radiograph and no evidence of left atrial hypertension or cardiogenic edema. ALI/ARDS is caused by a variety of pulmonary insults including severe influenza-like illness (ILI) due to COVID-19/SARS-CoV2, influenza, RSV, or other respiratory virus. ALI/ARDS is characterized by pulmonary edema/pneumonia, low blood oxygen level, poor lung compliance, severe lung inflammation, and sometimes increased systemic vascular permeability, shock, and sepsis. The overwhelming inflammatory response in ALI/ARDS involves elevated neutrophils and monocytes and the “cytokine storm” that damage not only lung surfactant but the airway epithelium including alveoli and their associated capillaries, compounding the original illness or injury. All-cause ARDS has a mortality rate in the 20-60% range. ARDS survivors often experience extended recovery periods with sometimes permanent loss of lung function and persistent symptoms. There are no approved treatments for ALI/ARDS, nor are there treatments to accelerate lung repair, reduce prolonged recovery periods, and reduce ARDS-associated permanent loss of lung function. COVID-19 is especially deadly because the virus destroys pulmonary epithelial cells and elicits a severe inflammatory response, resulting in a high rate of ALI/ARDS and a high mortality rate. Pulmonary edema and pneumonia occurs when inflammatory mediators increase vascular permeability, allowing serum to leak into extracellular pulmonary fluids and accumulate in pulmonary tissues. The presence of serum disrupts lung surfactant function, thereby reducing pulmonary compliance, mechanical lung function, and gas exchange. Lung surfactant is degraded by secretory phospholipase A2 enzymes (sPLA2s) that are upregulated and released during inflammatory responses and by reactive oxygen species (ROS) which damage surfactant phospholipids, inactivating lung surfactant and contributing to respiratory failure. COVID ARDS survivors often require ventilation for prolonged periods of time (1-2 months) and then experience extended recovery periods with persistent fatigue and other symptoms (“long COVID”), some with permanent loss of lung function. Survivors of ALI/ARDS, whether due to COVID-19 or not, typically undergo respiratory rehabilitation after hospital discharge in order to eliminate oxygen therapy, regain tolerance for exercise, and return to their normal daily activities. Native SCGB1A1 levels initially increase in ALI/ARDS because it is released by damaged Club cells. However, within hours to days SCGB1A1 is depleted and cannot be replaced because Club cells are also depleted. The rationale to use Therabron™ to prevent or treat ALI/ARDS is very strong in that Therabron™ mediates several biological activities through multiple mechanisms of action that are independent of the cause of the ALI/ARDS. Therabron™ is expected to benefit ALI/ARDS patients and a Phase 1 trial is planned using intravenously administered Therabron™.
|
Rationale for Use of TherabronTM in ALI/ARDS |
|
|
1 |
Preserves, promotes, and improves mechanical lung function/compliance (relieves at least certain types of bronchoconstriction) and blood gas exchange.
|
|
2 |
Reduces vascular permeability, pulmonary edema, systemic shock, normalizes fluid balance |
|
3 |
Stimulates epithelial repair, repopulates Club cells, and prevents pulmonary fibrosis in multiple lung injury models |
|
4 |
Neutralizes ROS in ALI/ARDS and simultaneously converts into more potent anti-inflammatory |
|
5 |
Inhibits inflammatory responses to viral infection or other injury
|
|
6 |
Inhibits viral replication in vitro, reduces viral titers in vivo |
|
7 |
Replaces and augments deficiencies of native SCGB1A1, caused by pre-existing conditions, genetic deficiency, age-related deficiency, and severe COVID-19 infection |
Asthma and Allergy
Asthma is a condition in which the airways react to inhaled substances, such as allergens, dust, and/or pollutants resulting in bronchoconstriction that limits air intake and can be life-threatening. Asthma may also be triggered by infection, cold, and exercise. Over 26 million Americans have asthma and over 4,000 Americans die from acute asthma attacks each year. Asthma is characterized by elevated airway inflammation and hyper-reactivity (AHR) that is typically treated with bronchodilators, beta-agonists, and corticosteroids. A subset of asthmatics with severe asthma characterized by airway eosinophilia are treated with the monoclonal antibody, Dupixent™. Over time, NF-B-dependent airway remodeling occurs in which Club cells and SCGB1A1 are depleted and airway fibrosis develops. Asthmatics are highly susceptible to experiencing more severe respiratory infections, including death from COVID-19. In the southwestern US, most cases of asthma are caused by infection with Mycoplasma pneumoniae, and recombinant human SCGB1A1 protein reversed the asthma phenotype in infected mice. Clinical studies have shown that asthmatics have fewer Club cells and lower levels of SCGB1A1 than those with normal lungs. Moreover, a specific genetic allele of the human SCGB1A1 gene that results in lower production of SCGB1A1 is associated with hereditary asthma. The rationale to use Therabron™ to prevent and treat asthma and allergy is very strong. Therabron™ has demonstrated the ability to reduce inflammatory innate immune responses (including reducing neutrophils, eosinophils, monocytes, and TH2 cytokines in the lungs), to reduce bronchoconstriction induced by viral and mycobacterial infection, methacholine challenge, and endotoxin exposure, and to reduce histopathology changes to the lungs in asthma models (ie. epithelial hyperplasia, mucus production, etc.). Several in vivo studies also showed that Therabron™ inhibits the development of allergen-specific TH2 cells in asthma and allergy models. Therefore, replacement and augmentation of native SCGB1A1 using inhaled Therabron™ is expected to control AHR, to restore pulmonary resistance to infection, to reduce inflammation and fibrosis in the airways of asthma patients, and to alleviate the severity of common nasal allergies.
COPD
Chronic obstructive pulmonary disease (COPD) is a debilitating, progressive condition involving airway inflammation and fibrosis accompanied by loss of lung function, primarily caused by cigarette smoking. Airway remodeling in COPD is associated with elevated NF-b activation. Annually, COPD affects 14 million Americans. The most common management strategy for COPD patients of all stages and severity involve the use of bronchodilators, which relieve bronchoconstriction and alters airway smooth muscle tone. Corticosteroids are often used to control inflammation and to prevent and treat exacerbations. This presents a treatment market expected to reach $14.1B by 2025, but for which the major drug class remains bronchodilators. Through investigation into the molecular and cellular mechanisms underlying COPD, including depletion of Club cells and SCGB1A1 protein, Therabron™ has been identified as a strong therapeutic candidate for COPD. However, bronchodilators and corticosteroids are inexpensive small molecule drugs compared to protein therapeutics such as Therabron™. Therefore, Therabron™ would most likely be used in a subset of COPD patients (~5-10%) that: 1) are not controlled with or cannot tolerate conventional COPD medications (ie. steroid-sparing treatment), 2) are severely deficient in native SCGB1A1 (<2 ng/mL in plasma or serum) leading to a precipitous loss of lung function and referred to as “rapid decliners”, and/or 3) are at higher risk of having low SCGB1A1 levels and developing COPD due to their SCGB1A1 genotype. The rationale for using Therabron™ to manage COPD is similar to that for its use in ALI/ARDS, asthma, and CLAD-BOS because many of the same underlying pathophysiological processes are impacted by Therabron™ in each of these conditions. Therefore, replacement and augmentation of native SCGB1A1 using inhaled Therabron™ is likely to preserve existing pulmonary function, to restore pulmonary resistance to infection and reduce inflammation and fibrosis in the airways of COPD patients thereby reducing the incidence and severity of COPD exacerbations, and to rehabilitate and accelerate recovery of those hospitalized for COPD exacerbations.
Transplant and Immune Modulation Applications
Lung inflammation and fibrosis can be caused by immune-mediated injury such as in allogeneic lung transplant (LTx) or hematologic cell (bone marrow) transplant (HCT). The exact cause and mechanisms of airway fibrosis in allogeneic LTx and HCT are unknown, however, progressive fibrosis and loss of lung function correlate with loss of Club cells and their major secretory product, the SCGB1A1 protein. SCGB1A1 and Therabron™ are known to reduce development of asthma and allergic rhinitis in response to specific allergen exposures in vivo. Not only does Therabron™ reduce innate immune responses, but also reduces T-cell and B-cell responses to specific antigens (TH1, TH1, and TH17). In particular, Therabron™, reduced the development of TH2 cells sensitive to tree pollen allergen in mixed lymphocyte reactions in vitro and to test allergen allergens in vivo. More recently, Trove and its collaborators determined that Therabron™ reduced titers of donor specific antibodies (DSA) in a murine model of orthotopic lung transplant model, as well as reducing specific T cell populations associated with the bronchiolitis obliterans phenotype. These exciting results suggest a potential to induce immunologic tolerance with potentially dozens of new applications for Therabron™ and its derivatives in many types of transplants, cell-based therapies, enzyme replacements, and other therapies that generate DSA, anti-drug antibodies, and T-cell- and B-cell-mediated immune responses in recipients, as well as in autoimmune diseases.
